University of Electro-Communications research: Combination imaging reveals fuel cell damage
Tokyo, Japan (PRWEB UK) 9 June 2015
A simultaneous view of both chemical distribution and bonding states in fuel cell membranes shows how and where irreversible degradation takes place. The newly published research results are an important advance to provide clean energy for powering vehicles.
Fuel cells have the potential to provide clean energy for powering vehicles, but improved performance and durability are needed for wide-spread commercialisation. A collaboration of researchers in Japan has now demonstrated a technique for simultaneously mapping the morphology as well as electronic and bonding states on fuel cell electrode membranes for the first time. The results show how the catalysts on the membrane electrodes degrade and provide insights for improving the durability.
Yasuhiro Iwasawa and colleagues from the University of Electro-Communications, the University of Tokushima and the Japan Synchrotron Radiation Research Institute studied proton exchange membrane fuel cells based on Nafion – an ion-containing polymer (ionomer) widely used for these devices. In their report of the results they point out how they might expect the non-uniform distribution of catalytic platinum nanoparticles to lead to non-uniform degradation throughout the fuel cell. As a result spatially resolved imaging of the membrane and catalytic platinum chemical species is key to determining how to reduce the deterioration of the catalyst, and hence improve the durability.
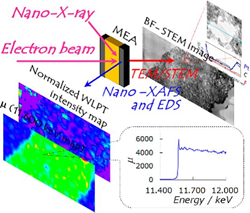
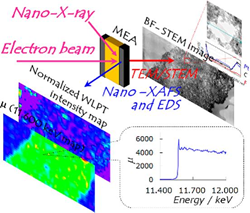
The researchers combined scanning transmission electron microscopy (STEM) and energy dispersive X-ray spectroscopy (EDS) techniques with X-ray absorption fine structure measurements (XAFS). “The STEM/EDS can give morphological information on atomic arrangement and element distribution, while the nano-XAFS can give molecular-level chemical information on electronic (oxidation) states and coordination structures with chemical bonding,” they explain in their report of the work.
By using their same-view STEM/EDS and XAFS equipment to compare the membranes before and after 300 cycles of gas exchange, they were able to identify two processes causing irreversible degradation of the platinum catalyst: detachment of the platinum nanoparticles from the carbon support and the formation of platinum ions. Further they found that these processes were dependent on the ratios of platinum and ionomer.
Background
Proton exchange membrane fuel cells
Fuel cells produce electrical energy from hydrogen and an oxidising agent such as oxygen or air. Catalysts cause the hydrogen molecules to break up into positive ions – that is, protons – and electrons. The proton exchange membrane allows the protons to pass through but the electrons follow an external circuit to the anode to complete the circuit, providing electricity (electric current).
Nafion was the first reported ion-containing polymer or ionomer. It has attracted a great deal of interest for fuel cells because of its high thermal and mechanical stability.
Electron microscopy
The resolving power of traditional optical microscopes is limited by the diffraction limit to around half the wavelength of the incident light. The resolving power of electron microscopes is much greater because the wavelength associated with the electron beams used is up to several orders of magnitude shorter than optical light.
Electron microscope images are derived from the changes in the electron beam after it is transmitted through the sample. By scanning the beam across the sample scanning transmission electron microscope (STEM) images are achieved. STEM images provide atomic-scale information about the shape and contours of a sample.
Energy dispersive x-ray spectroscopy
The electron beam can also stimulate the emission of x-rays from a sample. The wavelength of the x-rays emitted is determined by the atomic structure, which is specific to each individual element. As a result, measuring the peaks in the x-ray spectra – energy dispersive x-ray spectroscopy (EDS) – can identify the elements in STEM images.
X-ray absorption fine structure
When a sample is irradiated with a beam of x-rays, some pass through and some are absorbed at specific wavelengths depending on the binding energy of the electrons in the sample. Identifying these binding energies in this way gives an indication of the molecular-level chemical information on electronic (oxidation) states and coordination structures with chemical bonding.
Same-view cell
The researchers produced a ‘same-view’ membrane cell that allows the simultaneous view of both chemical distribution by STEM and bonding states by nano-XAFS in fuel cell membranes. By combining the information in these imaging techniques in the ‘same-view’ cell in this way they could monitor and locate the movement and ionisation of the catalytic platinum nanoparticles that deteriorates the fuel cell.
Reference
Title of paper: Same-View Nano-XAFS/STEM-EDS Imagings of Pt Chemical Species in Pt/C Cathode Catalyst Layers of a Polymer Electrolyte Fuel Cell.
Research publication: Journal of Physical Chemistry Letters, 2015, 6 (11), pp 2121–2126.
DOI: 10.1021/acs.jpclett.5b00750
Publication Date (Web): May 21, 2015
http://pubs.acs.org/doi/abs/10.1021/acs.jpclett.5b00750
Authors and Affiliations
Shinobu Takao,†Oki Sekizawa,†Gabor Samjeske,†Shin-ichi Nagamatsu,†Takuma Kaneko†Takashi Yamamoto,‡Kotaro Higashi,†Kensaku Nagasawa,†Tomoya Uruga,†,§and Yasuhiro Iwasawa*,†,∥
*Corresponding author
†Innovation Research Center for Fuel Cells, The University of Electro-Communications, Chofugaoka, Chofu, Tokyo 182-8585, Japan
‡Department of Mathematical and Material Sciences, Faculty of Integrated Arts and Sciences, The University of Tokushima,Minamijosanjima, Tokushima 770-8502, Japan
§Japan Synchrotron Radiation Research Institute, Spring-8, Sayo, Hyogo 679-5198, Japan
∥Department of Engineering Science, Graduate School of Information Engineering Science, The University of Electro-Communications, Chofugaoka, Chofu, Tokyo 182-8585, Japan
About The University of Electro-Communications
The University of Electro-Communications (UEC) in Tokyo is a small, luminous university at the forefront of applied sciences, engineering, and technology research. Its roots go back to the Technical Institute for Wireless Commutations, which was established in 1918 by the Wireless Association to train so-called wireless engineers in maritime communications in response to the Titanic disaster in 1912. In 1949, the UEC was established as a national university by the Japanese Ministry of Education, and moved in 1957 from Meguro to its current Chofu campus Tokyo.
With approximately 4,000 students and 350 faculty, UEC is regarded as a small university, but with particular expertise in wireless communications, laser science, robotics, informatics, and material science, to name just a few areas of research.
The UEC was selected for the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Program for Promoting the Enhancement of Research Universities as a result of its strengths in three main areas: optics and photonics research, where we are number one for the number of joint publications with foreign researchers; wireless communications, which reflects our roots; and materials-based research, particularly on fuel cells.
Website: http://www.uec.ac.jp/
Find More Physics Press Releases